AMD is one of the leading causes of vision loss among people aged 50 or above and can eventually lead to blindness in its advanced stages.
Current treatments for wet AMD help protect patients’ vision by restricting blood vessel growth that causes neovascularization; however, these medications require monthly injections into the eye.
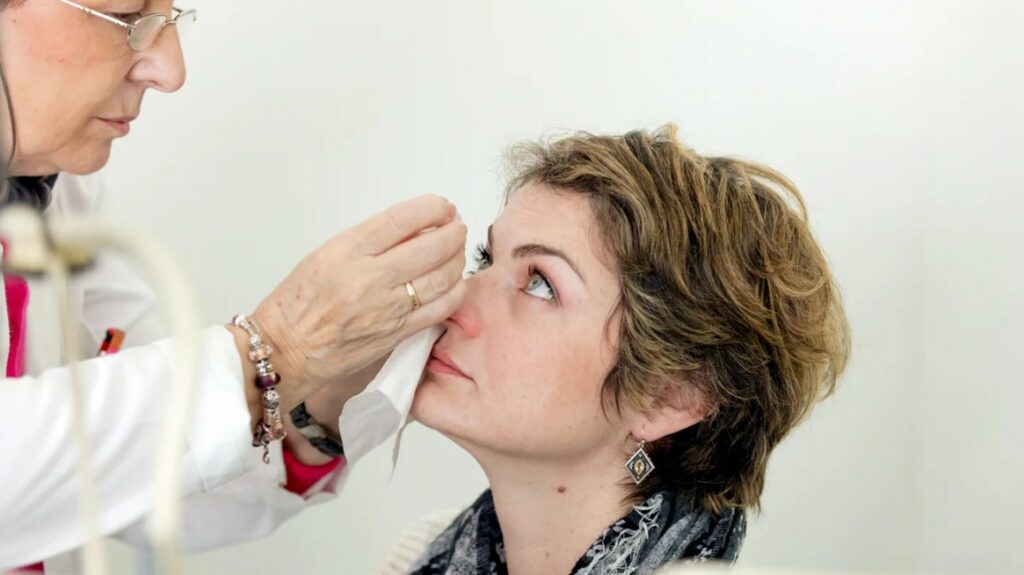
Intravitreal Injections
Each year in the US, millions of intravitreal injections are administered for various retinal diseases and conditions, performed by retinal specialists using various medications and delivery systems. They play an essential part in treating sight-threatening conditions like wet macular degeneration, diabetic retinopathy and retinal vein occlusion.
Injections into the vitreous fluid in the eye typically deliver medication directly to retinal tissues. They can also be used to treat certain types of retinal hemorrhage and reduce inflammation caused by retinal vein occlusions. Usually performed in-office under local anesthesia, patients usually return quickly back to their daily activities following this visit.
Retinal specialists carefully examine their patients before administering any medication injection, in order to make sure there are no areas of thinning (due to prior glaucoma surgery, for instance) near where medication could be injected and also identify retinal abnormalities which require either laser photocoagulation or monitoring.
There are currently many long-acting drugs under clinical trials for wet age-related macular degeneration that could help decrease injection frequency and office visits, such as Beovu (bevacizumab) which has been approved by the Food and Drug Administration to be injected directly into the eye to block activity of vascular endothelial growth factor, which stimulates blood vessel formation that contributes to macular degeneration.
Stem Cells
Stem cells are versatile cells with the potential to transform into different types of cells at will, providing researchers with a means to treat various conditions using stem cell treatments – from spinal cord injuries and Alzheimer’s to Parkinson’s, diabetes, heart disease and more.
Scientists recently demonstrated that human retinal pigment epithelium (RPE) stem cells can survive and integrate when transplanted into a patient with wet macular degeneration, making this breakthrough an important milestone in regenerative medicine that could result in off-the-shelf treatments for wet macular degeneration within five years.
People suffering from wet AMD who were given monthly injections of Zimura (avacincaptad pegol) combined with anti-VEGF improved their vision by approximately three lines on the Snellen chart. The treatment is currently undergoing phase two-b clinical trials, and could become available by 2018.
Scientists have also discovered that MDM2 inhibitors can effectively and safely decrease abnormal blood vessel formation in wet macular degeneration. A combined therapy approach of using both anti-VEGF vaccinations and an implant with MDM2 inhibitors to treat wet AMD may offer an effective long-term treatment option. Further research must be completed in order to evaluate its long-term safety.
Gene Therapy
Ophthalmologists from UC Davis Health recently employed gene therapy to successfully treat an individual suffering from wet age-related macular degeneration. This was the inaugural use of this treatment at UC Davis Eye Center and part of an ongoing clinical trial for potential new treatment solutions.
Research in this area seeks to develop an anti-VEGF medicine that the eye can produce on its own, using gene sequences given directly to patients that, once added to cells, cause them to start producing Aflibercept protein with minimal risk and complications.
Lucentis (aflibercept) is currently the only anti-VEGF medication approved to treat wet AMD, and requires monthly injections for maximum effectiveness. Unfortunately, vision gains tend to diminish with use over time and patient compliance may become challenging due to frequent visits for injections.
Regenxbio and AbbVie’s gene therapy trial aims to eliminate frequent injections with its innovative AAV approach for administering Aflibercept directly under retina. Animal studies have proven AAV injection to be safe, and so far, phase 2 trials with subretinal delivery as well as suprachoroidal delivery are taking place simultaneously.
Port Delivery System
The FDA recently granted approval to an implant that delivers anti-VEGF medication ranibizumab (Susvimo; Roche/Genentech), currently being investigated as a potential solution to diabetic macular edema and neovascular age-related macular degeneration treatment approaches. Archway phase 3 trial will explore long-term effectiveness versus intravitreal injections while simultaneously measuring its pharmacokinetics by tracking serum and aqueous humor levels of medication in subjects.
An ocular implant provides a steady dose of medication over months, making it an attractive solution for patients who do not wish to rely on monthly injections and may fear infections caused by needle-based procedures. The Ladder phase 2 clinical trial will demonstrate its safety by enrolling 240 participants diagnosed with wet macular degeneration who responded well to drug therapy.
Clinical trials of Susvimo’s ocular implant have demonstrated its efficacy and improvement of visual acuity compared to monthly intravitreal injections, although it does carry some risks; most importantly is endophthalmitis infection of the eye which occurred in 2.0% of implanted eyes versus 0.5% receiving monthly injections as reported in original studies of Susvimo; since then improved implant techniques and testing has reduced this risk; however new implantations have been suspended pending manufacturer review of this issue.
Abicipar
Clinical trials are underway with new long-acting anti-VEGF agents that could significantly decrease the need for monthly eye injections. By blocking vascular endothelial growth factor (VEGF), they prevent blood vessels from growing around and in the retina and protecting vision at risk without treatment – wet AMD patients currently receive an average of 13 injections annually.
Abicipar pegol (Allergan) is a small-molecule drug belonging to a new class of therapeutics known as DARPins, and in CEDAR and SEQUOIA phase III clinical trials was found to be at least as effective as ranibizumab (Lucentis, Genentech) with less frequent dosing among treatment-naive exudative AMD patients. MAPLE study included 123 participants over 50 years with active subfoveal/juxtafoveal/juxtafoveal/choroidal neovascularization due wet AMD who received either abicipar every eight weeks after three loading doses or ranibizumab monthly in double masked studies designed for comparisons against each other.
MAPLE results revealed that 93% of patients receiving abicipar were able to maintain stable vision over two years compared to only 80% receiving ranibizumab. While more severe instances were experienced when on abicipar, most were mild and treatable with topical anti-inflammatories. Allergan has developed an exclusive manufacturing process designed to remove inflammation-causing impurities from its Abicipar molecules; Allergan plans on using this material in future phase III trials; these results from MAPLE will also be presented at medical conferences.
RGX-314
RGX-314, a one-time gene therapy treatment designed to address wet age-related macular degeneration, diabetic retinopathy and other persistent retinal diseases, uses an NAV adeno-associated virus vector called AAV8 that encodes an antibody fragment to block vascular endothelial growth factor (VEGF), thought to cause leaky blood vessel growth that leads to accumulation of fluid within retina. REGENXBIO and AbbVie are testing this treatment in two phase 3 trials called ATMOSPHERE and AAVIATE respectively.
Atmosphere has already shown promise, achieving a non-inferiority margin of five ETDRS letters at one year. This trial aims to assess the safety and efficacy of suprachoroidal delivery of RGX-314 gene therapy in treating neovascular AMD with or without photodynamic therapy (PDT), with early results showing improved vision as well as significantly fewer rescue injections needed compared with patients receiving monthly ranibizumab injections as control injections.
AAVIATE is currently enrolling 465 patients who have neovascular AMD with occult lesion(s). Patients in cohort 1 will be randomly allocated either RGX-314 doses of either 6.4×1010 genomic copies per eye or 1.3×1011 genomic copies per eye compared with intravitreal injections of Aflibercept in ratio of 3:1; two other cohorts will also receive this medication, but at lower dosage levels while the third will include those refractory to PDT and negative for neutralizing antibodies against ranibizumab.