2022’s breakthrough treatments for dry macular degeneration feature several exciting clinical trials, ranging from gene therapy to non-steroid treatments for diabetic macular edema.
Faricimab-svoa (Vabysmo) was studied over two years, and showed promise for increasing vision in those suffering from neovascular age-related macular degeneration and visual impairment due to diabetic macular edema, while potentially decreasing injection frequency per year.
What is Syfovre?
On Friday, the Food and Drug Administration approved pegcetacoplan (Syfovre) for treating geographic atrophy (GA), an advanced form of age-related macular degeneration that can lead to vision loss. As a complement inhibitor, this drug slows leson growth caused by GA while simultaneously helping preserve vision by reducing more severe visual impairment or blindness.
GA is an irreversible eye disease characterized by the progressive breakdown of photoreceptor cells that produces non-hemorrhagic lesions that gradually cover the macula and eventually progress to blindness. This disease stands apart from wet or neovascular macular degeneration which involves leaky blood vessels growing inside retina, leading to permanent vision loss and eventually blindness.
Syfovre clinical trials revealed that both monthly and every-other-month injections significantly slowed geographical atrophy lesion growth compared to sham injection, but did not show significant differences in key visual function end points at 24 months. Furthermore, intraocular inflammation (IOI) measures were not statistically different between monthly and every-other-month groups in any trial; furthermore six cases of occlusive vascular occlusion occurred among Syfovre users as soon as seven days postinjection; one resulted in severe vision loss resulting in severe vision loss; no specific lot was implicated and their causes remain unknown.
Apellis released a statement noting its collaboration with ASRS and reviewing images to confirm that inflammation associated with Syfovre use is associated with using it, providing updates as they become available to retina specialists. Meanwhile, Molina recognizes Syfovre as medically necessary and will pay claims up until an updated J-code in CMS ASP Pricing File goes into effect; retina specialists may submit any appeals through CMS Part B Electronic Appeal System in the interim period.
How does Syfovre work?
Syfovre works by slowing the progression of geographic atrophy (GA), stabilizing vision loss in those with dry macular degeneration, and is administered on an ongoing or intermittent basis through intravitreal injection. Clinical trials showed it can slow vision decline for patients diagnosed with GA while simultaneously decreasing incidences of new GA lesions in treated eyes.
However, Syfovre may cause retinal vasculitis that leads to intraocular inflammation and vision loss, prompting the American Society of Retina Specialists (ASRS) recently to notify physicians of this potential risk from Syfovre use. So far six cases of occlusive retinal vasculitis have been reported by this ASRS notification to physicians 7-13 days post drug administration with no specific lots implicated.
Even with these rare adverse reactions, ASRS continues to recommend that doctors prescribe Syfovre to their patients. Syfovre announced in its latest update that it is investigating the possibility that one of its injection kits could have contributed to these symptoms, after discovering internal structural differences within 19-gauge filter needle variants present in some of its kits.
According to the company, these differences have not been proven as direct causes of retinal vasculitis in rare instances; rather they could be related to factors like improper injection technique, other medications or preexisting eye conditions. If such factors prove causal for retinal vasculitis episodes, all kits with 19-gauge filter needles should be immediately discontinued by practitioners.
Additionally, risks associated with this medication include the possibility of neovascular AMD or choroidal neovascularization developing in treated eyes – in fact this was observed in 13% of monthly injection arm patients and 7% of every other month injection arm patients in pivotal phase 3 DERBY and OAKS trials respectively. Furthermore, injection may temporarily increase vitreous floater numbers.
What are the side effects of Syfovre?
Keep a sharp lookout for any potential side effects associated with Syfovre use, including ocular discomfort, neovascular age-related macular degeneration, vitreous floaters and conjunctival hemorrhage. Although these side effects are common with intravitreal injections and affect patients of all ages; their appearance should not interfere with its benefits.
AMD affects over 16 million people in America and can lead to blindness over time, being the leading cause of vision loss among those aged 50+. It’s a progressive eye disease that attacks part of the retina known as the macula and impacts central vision, making it hard to drive, read or recognize faces over time. Dry age-related macular degeneration (dry AMD) is the most prevalent form of AMD; when parts of the macula become thinner or protein clumps called drusen start growing within it’s eye.
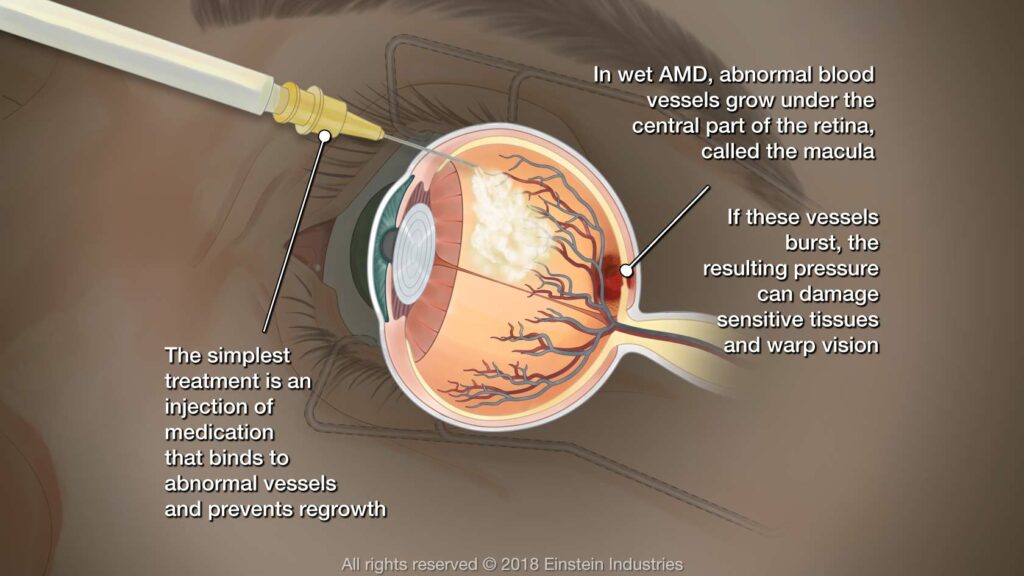
Wet age-related macular degeneration (wet AMD), however, occurs when new abnormal blood vessels form under the retina and start to leak blood or fluid, leading to scarring of the macula and rapid vision loss. Unfortunately, wet AMD can lead to permanent blindness unlike its dry counterpart.
Physicians had few treatment options available to them for treating late stage wet AMD; with the recent approval of pegcetacoplan, doctors now have access to an effective means of combatting this form of the condition.
Pegcetacoplan acts as a complement inhibitor, binding with high affinity to C2 and its activation fragment C3b as well as their subsequent cleavage to prevent downstream effectors of complement activation from being formed, and therefore decreasing complement-mediated inflammation in the eye.
Pegcetacoplan has been tested in clinical trials to assess its ability to slow the progression of wet AMD. According to pooled data from both DERBY and OAKS trials, patients treated with pegcetacoplan saw lower rates of new wet AMD than those given sham injections; although this may seem promising for practitioners, wet AMD may still worsen over time for many individuals despite these positive findings.
How much does Syfovre cost?
Syfovre injections cost approximately $2,190 each and were recently approved by the FDA as a treatment for geographic atrophy – a type of late-stage dry age-related macular degeneration (AMD). AMD leads to severe central vision loss for many over 65, becoming the leading cause of irreversible blindness in America.
Ophthalmologists and retina specialists administer this intravitreal injection, the first specifically approved to halt GA progression in those suffering with dry AMD. Furthermore, some patients report improved visual acuity while using this medicine – however not everyone sees an improvement in their vision.
However, it does not work in cases of neovascular or exudative AMD that involves bleeding into the retina and rapid loss of vision. Still, its FDA approval should give hope to patients suffering advanced dry AMD who have lost much of their vision due to progression of their condition.
Bevacizumab was recently approved by the FDA to treat both wet and dry forms of macular degeneration; however, according to a lawsuit brought forward by a former employee of its maker company, Bevacizumab has no effect in treating wet macular degeneration and has serious side effects that were not disclosed by its makers as risks or uncertainties were misrepresented by misrepresenting its effectiveness.
Silicone oil droplets found in the syringe used to administer drug may be responsible for mysterious eye floaters reported by some patients. According to research published in Molecular Therapy, researchers collected and analyzed eye floaters from Georgia Retina Specialists patients who received Syfovre injections using silicone oil lubricated with grease – likely from using this to lubricate their syringe.
Apellis Pharmaceuticals’ C3 inhibitor drug Syfovre was approved by the FDA in February 2023 as the only treatment currently available to slow geographic atrophy in patients suffering from dry macular degeneration. Acquisition is an attractive target given that wet macular degeneration treatment market is projected to expand significantly over time.