The main cause of blindness in elderly people is macular degeneration, often known as age-related macular degeneration (ARMD). According to estimates from 2004, 1.75 million Americans over the age of 40 suffer from ARMD, and in the next decades, that figure is likely to double. The incidence of ARMD differs by ethnicity. According to research by Wong et al., the pooled prevalence (age ranges from 45 years old to 85 years old) was 8.69%, with European descent showing the greatest prevalence (11.9%) and Asian descent showing the lowest prevalence (6.81%). According to a systematic analysis of European research, the prevalence of early ARMD is on the rise, going from 3.5% in the 55–59 age group to 17.6% in the >85 age group, while late ARMD prevalence goes from 0.1% in the 55–59 age group to 9.8% in the >85 age group. Therefore, aging is a key component in the emergence and progression of ARMD.
The most important factor in stopping the development of ARMD is age upon diagnosis. The development of imaging technologies has allowed for a detailed and thorough analysis of the ocular state even at the early stages of ARMD.
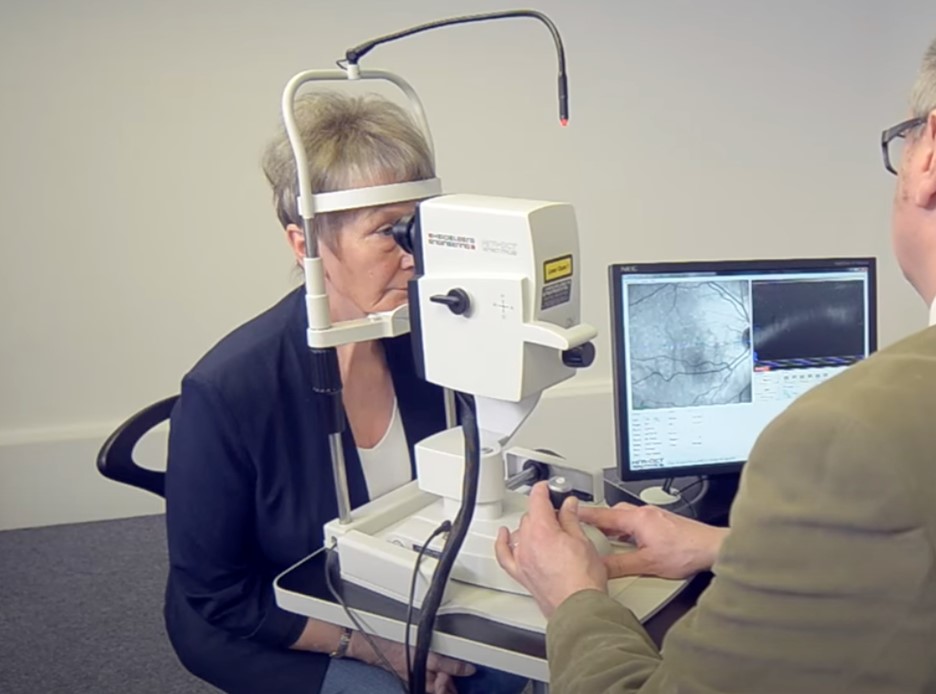
Retinal imaging has undergone a revolution in the last ten years, giving patients with age-related macular degeneration precise images of retinal abnormalities (AMD). Learn about the different retinal imaging techniques and how they aid eye physicians in detecting and treating AMD.
Optics, cameras, computers, and software developments have led to the development of new retinal imaging methods, which have shown to be very useful as diagnostic and therapeutic tools for AMD.
Because retinal imaging has become so important, AMD patients may anticipate having at least one kind of imaging taken and perhaps more on each visit to the eye doctor.
Techniques for retinal imaging that are used to identify and treat AMD a few examples:
- Fundus fluorescein angiography
- (ICGA) indocyanine green angiography
- Fundus photography
- Fundus autofluorescence
- (OCT) optical coherence tomography
- (OCTA) optical coherence tomography angiography
The advantages of each modality will be covered in more detail in this post.
A brief recap of age-related macular degeneration
Age-related macular degeneration is often divided into wet (exudative) and dry (nonexudative) ARMD based on these features. While this is happening, ARMD is divided into three stages: early, moderate, and advanced. 90% of instances of ARMD that have been diagnosed are dry cases. The presence of drusen buildup, the lack of choroid neovascularization, and retinal pigment epithelium (RPE) atrophy set this kind apart. The development of (CNV) choroidal neovascularization, in which delicate new blood vessels are prone to leakage and exudate formation, is the distinguishing feature of wet ARMD. It accounts for 10% of ARMD patients and has been connected to an early decline toward blindness.
The two constant risk factors associated with ARMD outside of age are smoking and ethnicity, which have been shown in research. Smoking doubles the likelihood of developing ARMD in 5 years compared to nonsmokers, according to a cohort study of people 65 years of age or older. ARMD’s precise underlying pathophysiology is still a mystery. There are a number of explanations put up as the underlying causes of ARMD. These include the buildup of lipofuscin, drusen, persistent inflammation, oxidative stress, a decline in antioxidants, complement mutation, and choroidal neovascularization. The development of the end-stage ARMD, which is exudative ARMD, is caused by the fragility of the new blood vessels, which leads to difficulties in the surrounding tissue that result in hemorrhages, exudate, RPE and/or retinal detachment, and scarring.
Delivering early therapy depends critically on the detection of ARMD in patients. Patients over 40 years old, in particular, are more likely to acquire ARMD. The American Academy of Ophthalmology recommends that people 40 years of age and older be tested for a potential ARMD. Drusen, severe symptoms of CNV such macular edema, subretinal fluid, hemorrhages, mottling or changes of the retinal pigment epithelium, and RPE atrophy must all be shown by a binocular slit-lamp examination using three mirror lenses or condensing lenses. It is advised that high-risk people have regular, thorough eye exams. Imaging techniques, both noninvasive and invasive, are used in a comprehensive eye exam to look for any small changes in the retinal structures.
AMD Progression
Our knowledge of the etiology and natural course of AMD has considerably increased as a result of multimodal imaging technological advancements. It is widely accepted that the early stages of AMD are frequently asymptomatic, have medium- and/or large-sized (soft) drusen, and have pigmentary abnormalities, despite a long-standing lack of agreement in the categorization of AMD. These eyes are at risk of developing neovascular AMD, which is characterized by choroidal or retinal neovascularization (NV) and concomitant significant vision loss, or GA (atrophy of the outer retina, RPE, and choriocapillaris).
It is now evident that non-neovascular AMD’s (Dry AMD) natural progression may exhibit significant variety, with several pathways leading to an unchanging result. One such approach is the collapse of massive soft drusen or a drusenoid PED or progressive outer retinal atrophy (ORA) coupled with reticular pseudodrusen and choroidal thinning. GA is, for instance, thought to be the end-stage form of non-neovascular AMD. When a long-term antiangiogenic medication has been administered to eyes with neovascular AMD, atrophy of the outer retina, RPE, and choriocapillaris may also be seen in such eyes.
The term AMD as it is now used refers to a collection of unique conditions that share clinical traits and downstream pathways. Our definition of AMD will be increasingly precise as our knowledge of the pathophysiologic and genetic pathways underlying the condition grows. This review paper focuses on the well-known non-neovascular AMD phenotype, which is notable for macular drusen of medium to large size, pigmentary anomalies (hyper- or hypopigmentation), and finally GA.
Traditional Dry AMD Classification
The imaging technologies available to detect features, such as macular drusen, RPE changes, and atrophy, has been a major factor in the development of classification schemes, severity scales, and grading systems for non-neovascular AMD. Numerous categorization methods depending on various imaging modalities have been presented throughout the years, but there hasn’t yet been a shared understanding.
Imaging modalities
Optical Coherence Tomography
In terms of retinal imaging, (OCT) optical coherence tomography has made the most strides . This method creates a cross-sectional image of the retina on a computer screen using infrared light reflections off the retina.
The photographs are captured quickly and without the use of any unpleasant light flashes. In patients with wet AMD, they reveal fluid pockets in the retina, which may be used to determine how often anti-VEGF injections (Beovu®, Eylea®, Lucentis®, or Avastin®) should be administered. Additionally, they exhibit retinal thinning brought on by cell death in individuals with late dry AMD as well as drusen, which are tiny deposits beneath the retina in early dry AMD patients (also called geographic atrophy).
Angiography using OCT
OCT angiography is a new development in OCT technology. This method makes it possible to view both normal and dysfunctional retinal blood vessels. This aids the ophthalmologist or optometrist in comprehending the kind and degree of irregular blood vessels present in AMD patients.
Before the development of OCT angiography, fluorescein or indocyanine green dye injections intravenously were the only way to acquire comprehensive pictures of retinal blood vessels (ICG). These examinations are more painful since they last 15 to 30 minutes and may include injections and light flashes. However, in order to have a clear and comprehensive picture of the disease process, tests are still sometimes necessary.
Fundus Photography
Photography of the retina or “fundus photography” offers a color image of the retina. Although this method has been around for a while, new optical technologies, digital cameras, computers, and software have all enhanced it. It simply needs a few quick, intense flashes and takes approximately a minute. Images collected may reveal scar tissue, regions where retinal cells have withered and faded away, or atrophy, and drusen, blood, or lipids (a group of naturally occurring compounds that includes fats) that have spilled out of irregular blood vessels in wet AMD.
Imaging using Autofluorescence
Autofluorescence imaging is another method for spotting atrophy. This approach provides a glimpse of the retinal pigment epithelial cells (RPE), which decrease in persons with geographic atrophy. It is very rapid and only needs a few brief bursts of light. RPE cells perform a variety of important tasks, including removing waste, nourishing, and protecting the retina.
Since dead RPE cells no longer contain lipofuscin, they show as a black patch in the image. Live RPE cells are bright because they contain the autofluorescent substance called lipofuscin.
Quantitative Autofluorescence
An internal fluorescent reference is used in a newly disclosed FAF technique to allow quantitative FAF while taking fluctuating laser intensity and detector sensitivity into consideration. This method may be constrained by media opacities and needs high-quality photos to get accurate quantitative data. Quantitative FAF characteristics have been shown by imaging of healthy eyes using this unique technology to rise with age, be greater in women, and perhaps vary by ethnicity. Although further research is required to confirm its use for AMD, this method has become more popular for investigating eyes with retinal dystrophies such as Stargardt disease. It may also be effective for evaluating regions of severe and nascent atrophy in AMD.
Color Fundus Autofluorescence
An innovative imaging technique called color FAF may provide fresh perspectives on retinal conditions including dry macular degeneration To separate the emission autofluorescence of lipofuscin in the RPE, FAF devices generally use a 488-nm wavelength excitation filter. Recent research has detailed a novel confocal blue-light FAF device (EIDON; CenterVue, Padua, Italy) that is believed to excite minor fluorophores that are different from lipofuscin and may give extra contrast to assess the macular structure. Due to the fact that the whole emission spectrum is measured on a color sensor and results in color FAF pictures, this novel method offers a potentially significant benefit. By separating the emission spectra into distinct short- and long-wavelength (green, 510-560 nm, and red, 560-700 nm) emission fluorescence components (EFC), it is possible to isolate minor fluorophores whose emission spectrum would normally be dominated by lipofuscin’s strong emission.
Using this color FAF system, a recent report of eyes with AMD atrophy showed that the strong red EFC from lipofuscin was absent or weak in areas of atrophy, while a residual green EFC signal was observed corresponding to subretinal hyperreflective material and was thought to represent drusenoid material partially made of advanced end products of glycation with fluorescent capability. To assess the predictive importance of these results and to further assess color FAF, larger longitudinal investigations are required.
Scanning Laser Ophthalmoscopy
A scanning laser ophthalmoscope (SLO) may provide an even sharper picture of the autofluorescent RPE cells, although this imaging requires a little more time and special tools. Adaptive optics-SLO (AO-SLO), which uses high-resolution telescope technology, may provide even greater resolution. This employs actual space-age technology that allows for images of and counts of individual retinal cells. At this time, AO-SLO is exclusively accessible in research settings.
For patients suspected of having retinal detachment or other peripheral retinal disorders, a relatively recent optical advancement in a camera developed by Optos offers a view of the peripheral retina in addition to the central retina (macula).
Conclusions
The main factor in slowing the course of advanced ARMD is the early detection of age-related macular degeneration. Without the necessity for an intrusive operation, the advancement in technology enables thorough anatomy of retinal change in ARMD. Early identification and thorough care of ARMD are outcomes of the use of multimodal imaging in ARMD diagnosis.
FAQ’s
What is the most diagnostic test for (AMD) macular degeneration?
An OCT is now probably the most used to initially diagnose macular degeneration.
Are Optometrists able to diagnose macular degeneration?
Macular degeneration is identified by ophthalmologists and optometrists as part of a thorough eye examination.
What kind of vision impairment may macular degeneration cause?
Among adults over 50, dry macular degeneration is a prevalent eye condition. It causes the macula to thin, which results in impaired or diminished central vision (MAK-u-luh). The portion of the retina called the macula is what gives you sharp vision when you look directly in front of you.
Are OCT scans safe for AMD?
Except for possible dryness or eye fatigue, optical coherence tomography scans have no known dangers or adverse effects. OCT is ineffective if you have thick cataracts or severe vitreous hemorrhage, however, since it depends on light to pass through the mediums.